Abstract

Introduction Recurrent bleeding in the same joint of a patient with hemophilia can lead to painful and chronic inflammation of the synovium, joint swelling, reduced range of motion, and in the long term joint arthropathy and irreversible joint damage. Early bleed resolution in the susceptible joint (known as a target joint) is thought to be crucial for preventing joint arthropathy and other incapacitating sequelae, and thus ultimately preserving joint function and health in persons with hemophilia.
Eptacog beta was FDA-approved in 2020 as a recombinant activated human factor VII for the treatment and control of bleeding episodes (BEs) in persons with hemophilia A or B and inhibitors (PwHABI) ages 12 years and older using two initial dose regimens (IDRs) of 75 or 225 µg/kg eptacog beta that were shown to be safe and efficacious. The pivotal phase 3 trial (PERSEPT 1; NCT02020369) included 27 PwHABI ages 12 to 54 years who treated 465 mild or moderate BEs, with treatment success proportions at 12 hours post-initial infusion of 82% and 91% for 75 and 225 µg/kg IDRs, respectively.
Objective To evaluate eptacog beta efficacy when treating target and non-target joint BEs in PwHABI from PERSEPT 1, at 12 and 24 hours post-initial dose of eptacog beta.
Methods PERSEPT 1 was a global, multicenter, open-label, prospective phase 3 trial using a crossover design (Wang et al., Haemophilia 23, pp. 832-43 [2017]). PwHABI who were not using prophylaxis were randomized to either the 75 or the 225 µg/kg IDR to treat BEs, and were crossed over to the alternate IDR every 3 months. Subjects in the 75 µg/kg IDR treated BEs with initial doses of 75 µg/kg eptacog beta followed by 75 µg/kg q3h as needed. Subjects in the 225 µg/kg IDR treated BEs with an initial 225 µg/kg eptacog beta dose, followed after 9 hours by 75 µg/kg q3h as needed. Mild or moderate BE treatment success at a given timepoint was defined as obtaining a hemostasis evaluation of "excellent" or "good" on a 4-point scale ("excellent" or "good" indicating hemostatic efficacy; "moderate" or "none" indicating lack of efficacy) with no additional eptacog beta being infused, no alternative hemostatic agents or blood products being used, and no increase in pain following the first "excellent" or "good" assessment. A target joint was defined in this trial as a joint in which recurrent bleeding had occurred on 4 or more occasions during the previous 6 months, or a joint in which 20 lifetime BEs had occurred.
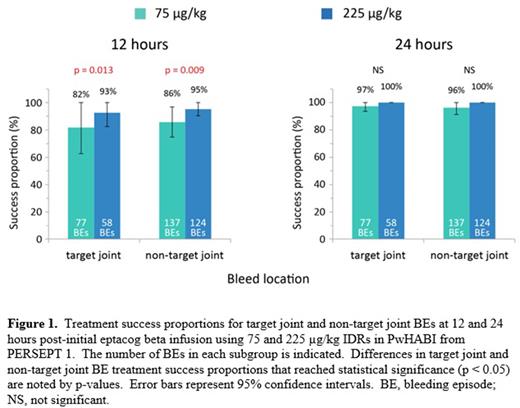
Results Twenty-seven PwHABI treated 396 mild or moderate joint BEs in PERSEPT 1; 17 of these subjects had been previously diagnosed with target joints. Of the 396 mild or moderate joint BEs treated, 135 were target joint bleeds (in 15 subjects) and 261 were non-target joint bleeds (in 25 subjects). At 12 hours post-initial infusion, target joint treatment success proportions and 95% confidence intervals (CIs) were 82% (95% CI: [63%, 100%]) and 93% (95% CI: 82%, 100%]) in the 75 and 225 µg/kg IDRs, respectively (Figure 1). Corresponding success proportions and CIs for non-target joint bleeds were 86% (95% CI: [75%, 97%]) and 95% (95% CI: [90%, 100%]), respectively. The increase in treatment success proportion observed at 12 hours with the 225 µg/kg IDR over the 75 µg/kg IDR was statistically significant for both target joint and non-target joint groups (p < 0.05). For both IDRs, the difference in treatment success proportions between target and non-target joint BEs was not statistically significant. Nearly all target (97-100%) and non-target joint bleeds (96-100%) were successfully treated at 24 hours post-initial eptacog beta infusion in each IDR (Figure 1).
Conclusions The increase in both target and non-target joint bleed treatment success seen at 12 hours for the 225 µg/kg IDR over the 75 µg/kg IDR was statistically significant, and is consistent with a dose-dependent thrombin burst driving effective clot formation at the site of injury. While the 75 µg/kg IDR may be appropriate for certain patients, the 225 µg/kg IDR shows improved treatment success at 12 hours and may be the preferred choice in most circumstances. With the high efficacy seen at 24 hours, eptacog beta offers an important treatment option for treating both target and non-target joint BEs in PwHABI.
Disclosures
Reding:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Hema Biologics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Biomarin: Consultancy, Research Funding; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: medical writing support, Research Funding, Speakers Bureau. Ahuja:Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; ClotChip: Patents & Royalties; TraumaChek: Patents & Royalties; State of Ohio Rare Disease Advisory Council: Membership on an entity's Board of Directors or advisory committees. Chitlur:NovoNordisk: Consultancy, Honoraria; Agios Pharmaceuticals: Honoraria, Research Funding; BPL Inc: Honoraria; Emerging Therapeutics Inc: Honoraria; Genentech Pharmaceuticals: Honoraria, Research Funding; Sanofi/Genzyme Corp: Honoraria. Dunn:Genentech: Consultancy; Kedrion: Consultancy; CSL Behring: Consultancy; Biomarin: Consultancy, Research Funding; Takeda: Research Funding; Freeline: Research Funding; Novo Nordisk: Research Funding; Sanofi: Research Funding; American Society of Thrombosis and Hemostasis: Research Funding; World Federation of Hemophilia, USA: Membership on an entity's Board of Directors or advisory committees. Escobar:Genentech: Honoraria; CSL Behring: Honoraria; Bayer: Honoraria; Takeda: Honoraria; Novo Nordisk: Honoraria; UniQure: Honoraria; Sanofi: Honoraria; Kedrion: Honoraria; Hemobiologics/LFB: Honoraria; The National Hemophilia Foundation: Honoraria; Pfizer: Honoraria; BioMarin: Honoraria. McGuinn:BPL: Consultancy; CSL Berhing: Membership on an entity's Board of Directors or advisory committees; Genentech/ Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; HemaBiologics: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Spark Therapeutics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding. Sidonio, Jr.:Novo Nordisk: Consultancy; Genentech: Consultancy, Research Funding; Biomarin: Consultancy; Catalyst: Consultancy; Sobi: Consultancy; Sanofi: Consultancy; Takeda: Consultancy, Research Funding; Octapharma: Consultancy, Research Funding; Bayer: Consultancy; Pfizer: Consultancy; Grifols: Consultancy, Research Funding; Kedrion: Consultancy; HEMA Biologics: Consultancy. Wang:Takeda: Consultancy, Other: principal investigator; Genentech: Consultancy, Other: principal investigator; Novo Nordisk: Consultancy, Other: principal investigator; CSL Behring: Consultancy, Other: principal investigator; Bioverativ: Consultancy, Other: principal investigator; Bayer: Consultancy, Other: principal investigator; BioMarin Pharmaceutical Inc.: Consultancy, Other: principal investigator; Pfizer/Spark: Consultancy, Other: principal investigator; Octapharma: Consultancy, Other: principal investigator; uniQure: Consultancy, Other: principal investigator; HEMA Biologics: Consultancy, Other: Clinical trial investigator. Windyga:Alnylam: Research Funding; Baxalta: Honoraria, Research Funding; Novo Nordisk: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; Rigel Pharmaceuticals: Research Funding; Roche: Honoraria, Research Funding; Shire/Takeda: Honoraria, Research Funding; Sobi: Honoraria, Research Funding; Alexion: Honoraria; CSL Behring: Honoraria; Ferring Pharmaceuticals: Honoraria; LFB: Honoraria; Sanofi/Genzyme: Honoraria; Siemens: Honoraria; Werfen: Honoraria. Wang:LFB-USA, Inc.: Current Employment. Wilkinson:GLOVAL, LLC: Consultancy. Pipe:CSL Behring: Consultancy; Apcintex: Consultancy; Bayer: Consultancy; ASC Therapeutics: Consultancy; Freeline: Consultancy; HEMA Biologics: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; Spark Therapeutics: Consultancy; UniQure: Consultancy; Regeneron/Intellia: Consultancy; BioMarin Pharmaceutical Inc.: Consultancy; Novo Nordisk: Consultancy; Sangamo Therapeutics: Consultancy; Sanofi: Consultancy; Takeda: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal